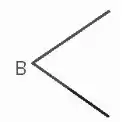
Câu hỏi:
Điền số thích hợp vào ô trống:

Ở đồng hồ trên, hai kim tạo thành góc...........độ.
Trả lời:
Đáp án đúng: C
Ở đồng hồ trên, hai kim tạo thành góc 120 độ.
Câu hỏi này thuộc đề thi trắc nghiệm dưới đây, bấm vào Bắt đầu thi để làm toàn bài
Bộ Đề Kiểm Tra Giữa Học Kì I - Toán 4 - Chân Trời Sáng Tạo - Bộ Đề 02 giúp học sinh ôn tập các kiến thức trọng tâm như số tự nhiên, bốn phép tính, toán có lời văn, yếu tố hình học và đo lường. Đề gồm cả trắc nghiệm và tự luận, phù hợp chuẩn kiến thức – kĩ năng, hỗ trợ học sinh rèn luyện và giáo viên, phụ huynh tham khảo.
29/09/2025
0 lượt thi
0 / 22
Câu hỏi liên quan

Trọn Bộ Giáo Án Word & PowerPoint Tiếng Anh 12 – I-Learn Smart World – Năm Học 2025-2026

Trọn Bộ Giáo Án Word & PowerPoint Tiếng Anh 12 – Global Success – Năm Học 2025-2026

Trọn Bộ Giáo Án Word & PowerPoint Hóa Học 12 – Kết Nối Tri Thức – Năm Học 2025-2026

Trọn Bộ Giáo Án Word & PowerPoint Hóa Học 12 – Chân Trời Sáng Tạo – Năm Học 2025-2026

Trọn Bộ Giáo Án Word & PowerPoint Công Nghệ 12 – Kết Nối Tri Thức – Năm Học 2025-2026